Research
Epigenetic and Transcriptional Regulation Governing Mesenchymal Stem Cell Differentiation
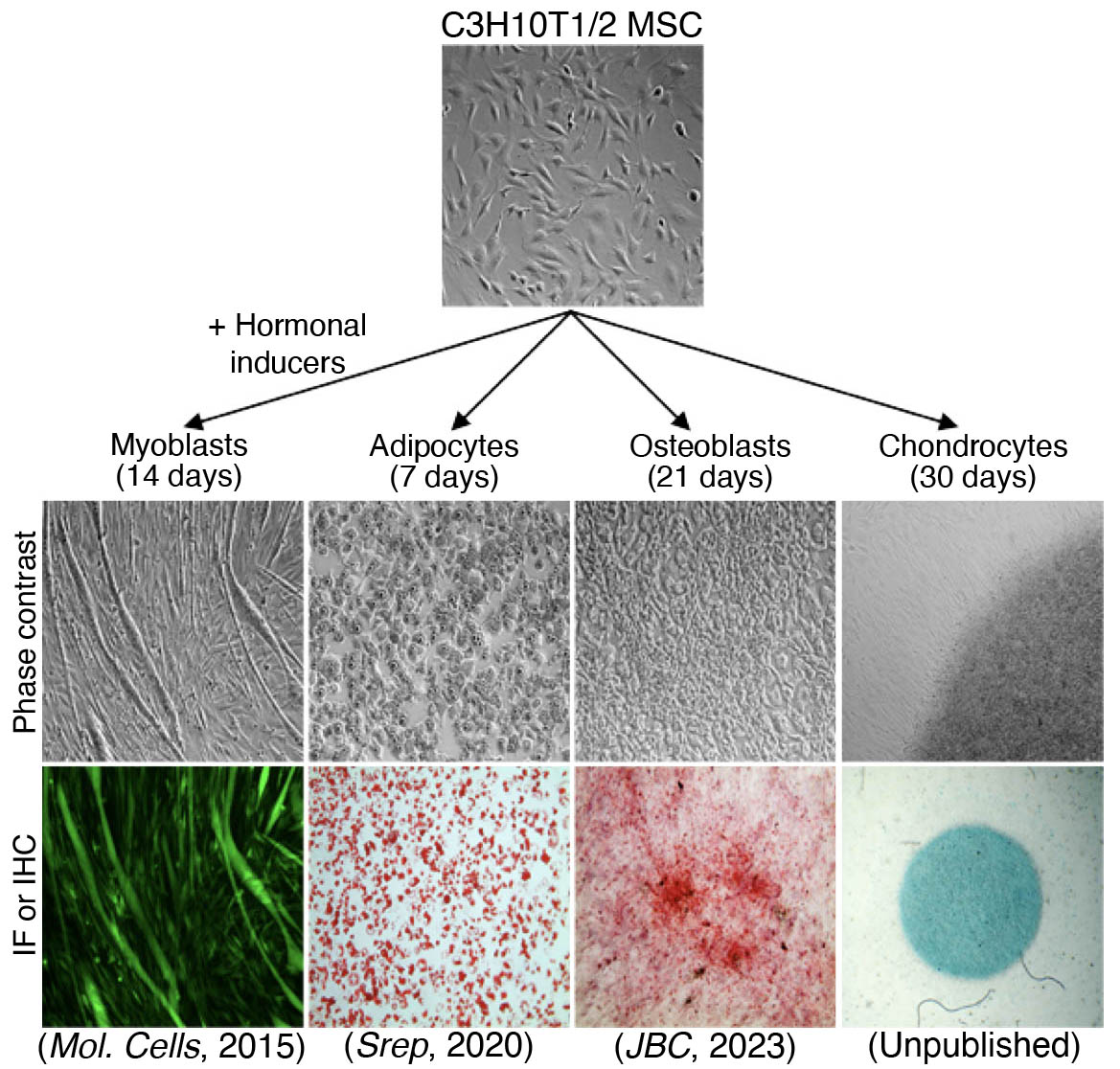
Histone modifier enzymes cooperate with lineage-specific transcription factors, playing a pivotal role in development and differentiation. Delving deeper, histone acetylation often represents gene activation, while histone methylation can indicate either activation or repression, depending on the specific methylation degree at a particular lysine (K) residue. For instance, histone tri-methylation at H3K4 (H3K4me3) is related to gene activation, whereas histone tri-methylation at H3K9 (H3K9me3) and H3K27 (H3K27me3) is associated with gene repression. To discover the complex network of epigenetic regulation in cell fate determination, we turned to mesenchymal stem cell (MSC) differentiation—spanning myogenesis, adipogenesis, and osteogenesis—using mouse MSCs as our model system. We have shown;
- SETDB1, an H3K9-specific methyltransferase, is required for the maintenance of MyoD, a master regulator of myogenesis, in proliferating mouse C2C12 myoblast cells (Mol. Cells, 2015), while the H3K9-specific demethylase KDM4B interacts with MyoD to regulate the expression of myogenic regulatory factors, including Myog and the Myod1 gene itself (BBRC, 2015).
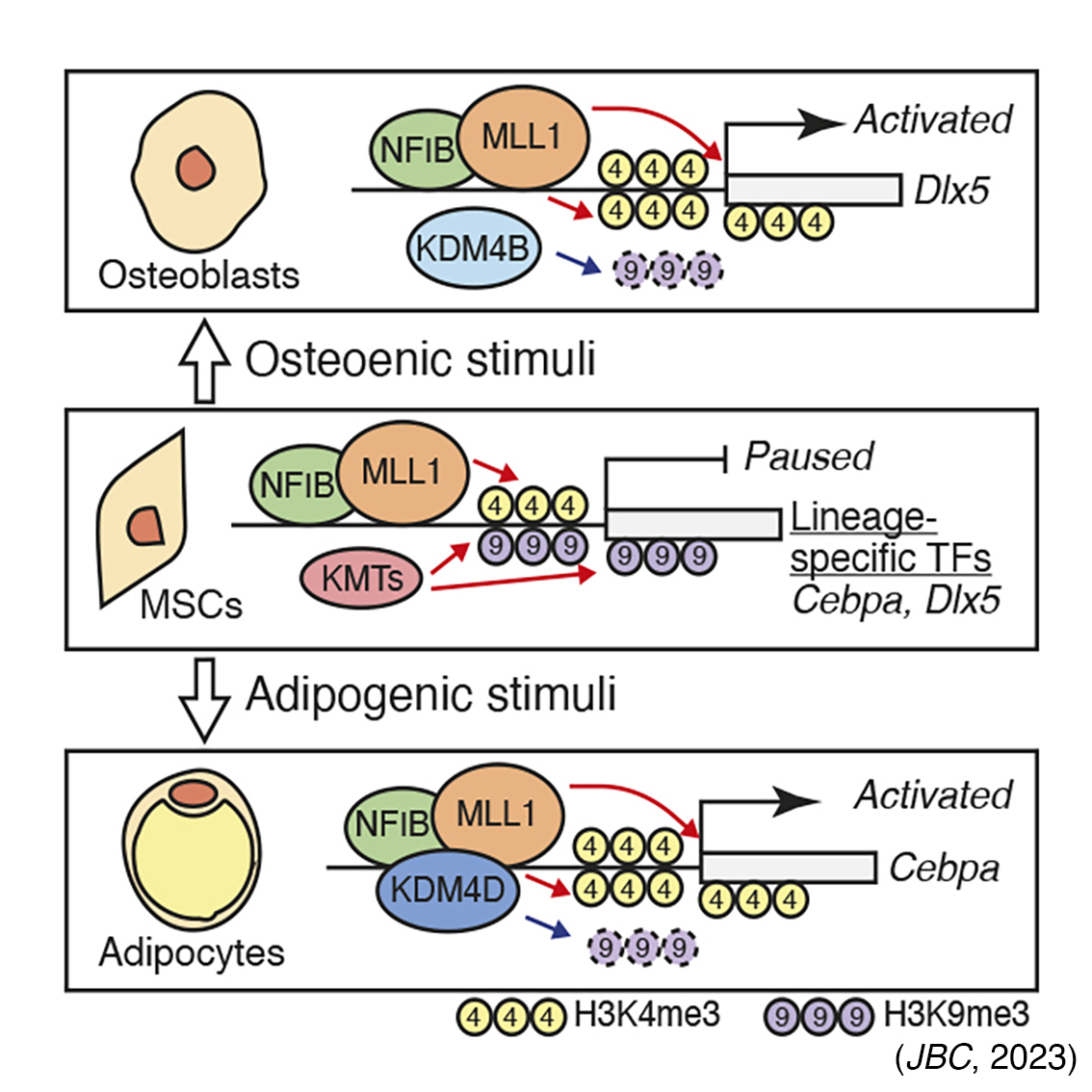
- KDM4D, an H3K9-specific demethylase, is pivotal in the adipogenesis of mouse C3H10T1/2 MSCs by removing repressive histone marks at the promoters of Pparg and Cebpa through its interaction with NFIB, a nuclear transcription factor, and the H3K4-specific methyltransferase MLL1 (Srep, 2020), and, in conjunction with the NFIB/MLL1 complex, it also targets critical developmental genes such as Dlx5 and Cebpa, thereby establishing a chromatin landscape characterized by H3K4me3/H3K9me3 in uncommitted MSCs (JBC, 2023).
Epigenetic Insights into the Regulation of Cytoprotection against Oxidative Stress
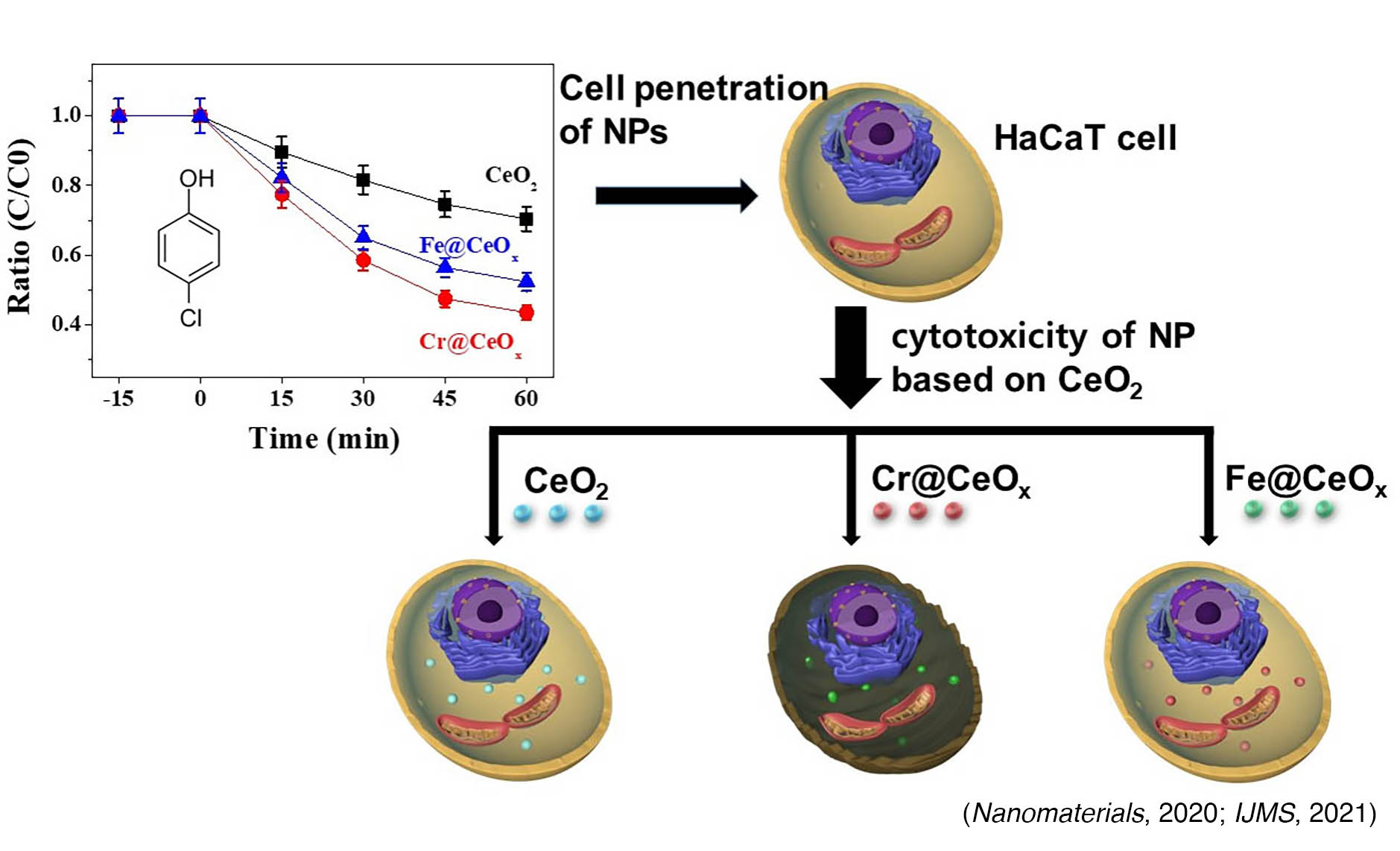
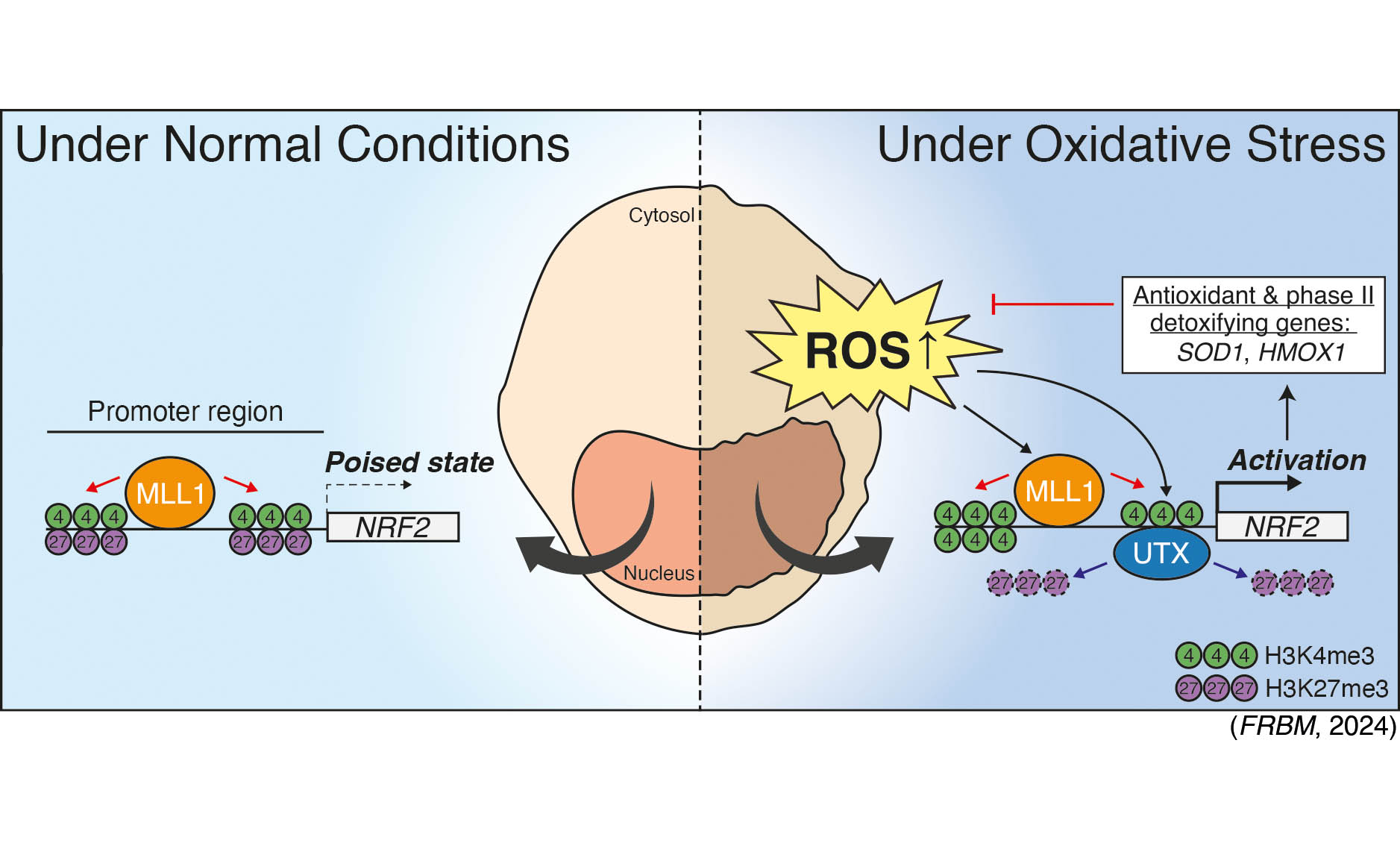
Cells frequently face the challenge of oxidative stress, which triggers an increase in intracellular reactive oxygen species (ROS). This elevation in ROS can decrease cell viability and contribute to the onset of cellular senescence, a recognized hallmark of aging. The NRF2-KEAP1 signaling is known as a vital pathway in counteracting oxidative stress and in regulating redox homeostasis. To explore this further, we employed various ROS generators such as H2O2, menadione, and tBHP, in conjunction with transition metal-doped cerium oxide nanoparticles (TM@CeO2 NPs). We utilized human keratinocytes as our model system to investigate the epigenetic mechanisms of cytotoxicity, particularly focusing on the NRF2-KEAP1 pathway. We have shown;
- TM@CeO2 (TM = Cr, Mn, Fe, Co, or Ni) NPs exhibit enhanced photocatalytic performances, however, only Cr-doped CeO2 NPs (Cr@CeO2 NPs) not only prompt severe negative effects on the viability of human HaCaT cells (Nanomaterials, 2020) but also disturb the deposition of H3K4me3 by MLL1 at the NRF2 promoter, leading to the failure of cells to activate the NRF2-KEAP1 pathway (IJMS, 2021).
- MLL1 and UTX, an H3K27-specific demethylase, functionally cooperate to establish a chromatin environment that favors active transcription at the H3K4me3/H3K27me3 bivalent NRF2 promoter in response to ROS-induced oxidative stress (FRBM, 2024).